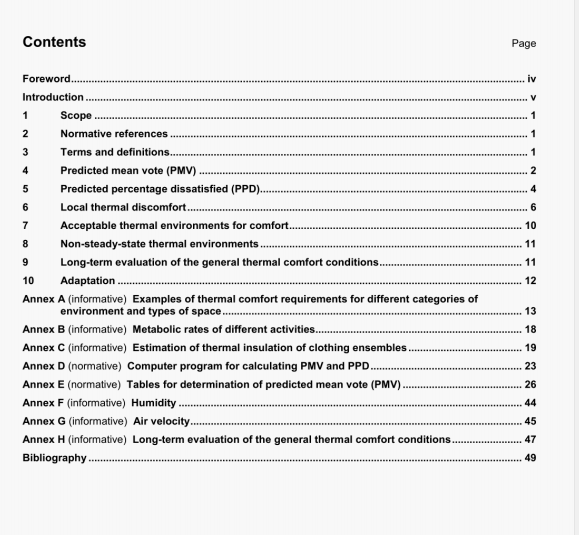
ISO 12830:2011 pdf download.Paper, board and pulps- Determination of acid-soluble magnesium, calcium,manganese, iron, copper, sodium and potassium.
NOTE This cesium solution is used to suppress ionization of sodium and potassium It is also used to suppress ionization of calcium in a nitrous oxideacetylene flame. The solution is not requwed when the air/acetylene flame or ICP technique is used
4.6 Standard stock solutions of each element, commercially available cerithed atomic absorption or atomic emission standard solutions can be used. Standard stock solutions can also be prepared as follows:
4.6.1 MagnesIum, 1 000 mg/i standard solution. Dissolve 1.000 g of magnesium metal ribbon in 100 ml of
1:4 nitric acid (4.3) and dilute to 1 000 ml with water,
4.62 Calcium, 1 000 mg/I standard solution. Dissolve 2.4979 of primary standard calcium carbonate (CaCO3) in a minmium volume of 1:4 nItric acid (4.3) and dilute to 1 000 ml with water.
4.6.3 Manganese. 1 000 mg/i standard solution. DIssolve 1.000 g of manganese metal strip or wire In a minimum volume of 1:1 nitric acid (4.3) and dilute to 1 000 nil with water,
4.6.4 Iron, 1 000 mg/i standard solution. Dissolve 1.000 g of iron metal stflp or wire in 20 ml of hydrochloric acid (4.2) and dilute to 1 000 ml with water.
4.6.5 Copper, 1 000 mg/I standard solution. Dissolve 1.000 g of copper metal stnp or wire in a minimum volume of 1:1 nitric acid (4.3) and dilute to 1 000 ml with water
4.6.6 Sodium, 1 000 mg/I standard solution. Ignite a portion of anhydrous sodium sulfate (Na2SO4) at 550 C in a crucible of platinum or porcelain. Allow to cool to room temperature in a desiccator. Dissolve 3.0899 of dried sodium sulfate in water and dilute to 1 000 ml with water, Store in a polyethylene bottle.
4.6.7 Potassium 1 000 mg/i standard solution. Ignite a portion of anhydrous potassium sulfate (K2S04) at
550 t in a crucible of platinum or porcelain. Allow to cool to room temperature in a desiccator. Dissolve 2.2289 of dried potassium sulfate in water and dilute to 1 000 ml with water, Store in a polyethylene bottle.
4.7 Acetylene gas and/or nitrogen oxide gas, of a grade suitable for atomic absorption spectrometry. Nitrous oxide is used only when measuring calcium.
WARNING — Acetylene gas forms explosive mixtures with air.
4.8 Carrier gas. appropriate gas for the plasma emission spectrometer. Argon is usually recommended as a carner gas.
4.6 Standard stock solutions of each element, commercially available cerithed atomic absorption or atomic emission standard solutions can be used. Standard stock solutions can also be prepared as follows:
4.6.1 MagnesIum, 1 000 mg/i standard solution. Dissolve 1.000 g of magnesium metal ribbon in 100 ml of
1:4 nitric acid (4.3) and dilute to 1 000 ml with water,
4.62 Calcium, 1 000 mg/I standard solution. Dissolve 2.4979 of primary standard calcium carbonate (CaCO3) in a minmium volume of 1:4 nItric acid (4.3) and dilute to 1 000 ml with water.
4.6.3 Manganese. 1 000 mg/i standard solution. DIssolve 1.000 g of manganese metal strip or wire In a minimum volume of 1:1 nitric acid (4.3) and dilute to 1 000 nil with water,
4.6.4 Iron, 1 000 mg/i standard solution. Dissolve 1.000 g of iron metal stflp or wire in 20 ml of hydrochloric acid (4.2) and dilute to 1 000 ml with water.
4.6.5 Copper, 1 000 mg/I standard solution. Dissolve 1.000 g of copper metal stnp or wire in a minimum volume of 1:1 nitric acid (4.3) and dilute to 1 000 ml with water
4.6.6 Sodium, 1 000 mg/I standard solution. Ignite a portion of anhydrous sodium sulfate (Na2SO4) at 550 C in a crucible of platinum or porcelain. Allow to cool to room temperature in a desiccator. Dissolve 3.0899 of dried sodium sulfate in water and dilute to 1 000 ml with water, Store in a polyethylene bottle.
4.6.7 Potassium 1 000 mg/i standard solution. Ignite a portion of anhydrous potassium sulfate (K2S04) at
550 t in a crucible of platinum or porcelain. Allow to cool to room temperature in a desiccator. Dissolve 2.2289 of dried potassium sulfate in water and dilute to 1 000 ml with water, Store in a polyethylene bottle.
4.7 Acetylene gas and/or nitrogen oxide gas, of a grade suitable for atomic absorption spectrometry. Nitrous oxide is used only when measuring calcium.
WARNING — Acetylene gas forms explosive mixtures with air.
4.8 Carrier gas. appropriate gas for the plasma emission spectrometer. Argon is usually recommended as a carner gas.